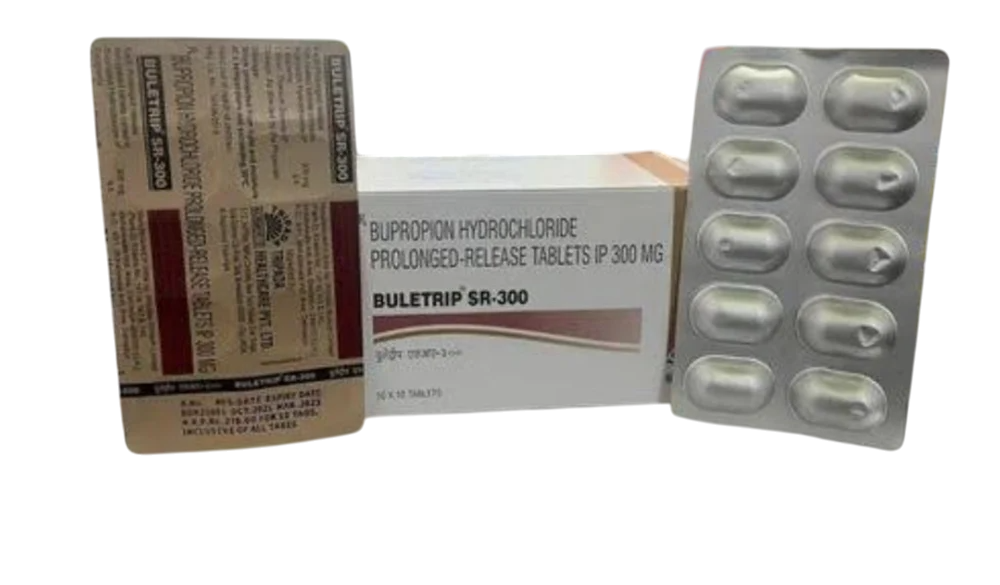
Buletrip SR 300 Mg (Bupropion)
Buletrip SR 300 mg (Bupropion) is a sustained-release antidepressant used in the treatment of major depressive disorder (MDD), seasonal affective disorder (SAD), and as an aid for smoking cessation. Belonging to the norepinephrine-dopamine reuptake inhibitor (NDRI) class, it helps regulate brain chemicals such as dopamine and norepinephrine, which play a crucial role in mood, motivation, and concentration.
Unlike many other antidepressants, Bupropion is less likely to cause weight gain or sexual side effects, making it a preferred choice for many patients. The sustained-release (SR) formulation allows for a gradual release of medication, providing long-lasting therapeutic effects while minimizing side effects.
Key Benefits:
-
Relieves symptoms of depression and low mood
-
Helps reduce cravings and withdrawal symptoms during smoking cessation
-
Improves energy, focus, and motivation
-
Lower risk of sedation compared to other antidepressants
Usage:
Buletrip SR 300 mg should be taken exactly as prescribed by a healthcare professional, usually once or twice daily, with or without food. Dosage adjustments may be required based on the patient’s condition and response to treatment.
Safety Note: This medication should not be used in patients with a history of seizures, eating disorders, or alcohol withdrawal. Common side effects may include insomnia, dry mouth, headache, or anxiety. Always consult a doctor before starting or stopping treatment.
| Quantity |
|---|






Reviews
There are no reviews yet.